|
IPIM
|
Module computing chemistry. More...
Public Member Functions | |
| subroutine | chemistry (ion_n, neutre, ialt, itube) |
| Inspired from reac_fillmatrix. More... | |
| subroutine | compute_rrate (rtype, ncoeff, coeff, T, P, k_r) |
| Calculates the reaction rate for a given reaction type. More... | |
| subroutine | get_third_spec (sp, ptr, nnr, nr, nnu, nu, nc, idx) |
| Gives the concentration of the third reactant in a 3-species reaction. More... | |
| subroutine | print_bilan_reaction (fid, sp, re, ptr, x_kr, x_n1, x_n2, x_to, x_T) |
Module computing chemistry.
Module computing chemistry
| subroutine module_chimie::chemistry | ( | type(espece), dimension(:), intent(in) | ion_n, |
| real*8, dimension(:), intent(in) | neutre, | ||
| integer, intent(in) | ialt, | ||
| integer, intent(in) | itube | ||
| ) |
Inspired from reac_fillmatrix.
| [in] | itube | Local temperature |
| [in] | itube | Local pressure |
| [in] | itube | Concentrations of the resolved species |
| [in] | itube | Concentrations of the non resolved species |


| subroutine module_chimie::compute_rrate | ( | rtype, | |
| ncoeff, | |||
| coeff, | |||
| T, | |||
| P, | |||
| k_r | |||
| ) |
Calculates the reaction rate for a given reaction type.
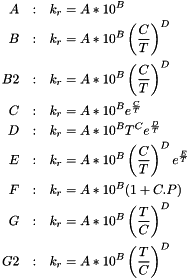

| subroutine module_chimie::get_third_spec | ( | type(spec) | sp, |
| type(one_reaction), pointer | ptr, | ||
| nnr, | |||
| nr, | |||
| nnu, | |||
| nu, | |||
| nc, | |||
| idx | |||
| ) |
Gives the concentration of the third reactant in a 3-species reaction.
| sp | Pointer to the current species list |
| ptr | Pointer to the current reaction |
| ptr | Number of reactants in the reaction |
| ptr | Number of species resolved in the code |
| ptr | Number of species non-resolved (given by a model) |
| ptr | Concentations of the resolved species |
| ptr | Concentration of the non-resolved species |
| ptr | Ouput value [ 1:No 3rd reactant | nc:concentration of the 3rd reactant ] |
| ptr | Index of the 3rd reactant |

 1.8.5
1.8.5